A number of people have already filed Zimmer Hip Lawsuit against
orthopedics manufacturing company Zimmer, whose responsibility for the hip
replacement device known as the Durom Cup has seen many people turn their
attention in the company’s direction—but most of the press is negative.
Democrats on the U.S. House Committee on Energy and Commerce are getting
involved—questioning why harmful brain stents and metal-on-metal Zimmer hip replacement recall
continue to be sold throughout the United States with little oversight.
Metal-on-metal hip replacement systems like
the Zimmer Durom cup come in a variety of models from a variety of
manufacturers but often side effects are the same. Component loosening, soft
tissue damage, bone fracture, metal poisoning (metallosis), premature device
dislocation, and Zimmer
hip revision surgery are all directly linked to metal-on-metal hip
replacement systems. This is worrisome to some House Democrats, who hope to
convince Republicans that this issue needs to be looked into with greater
depth.
Zimmer patients who have experienced severe
side effects already know the issues that brought the devices to the attention
of a part of the United States’ government. Zimmer actually recalled the Durom
cup for a short time, claiming that they fixed the problems associated with it,
but patients are still skeptical—especially those who have had to undergo hip
revision surgery, a complex procedure that comes with a number of its own
risks.
Those who have experienced side effects of
metal-on-metal hip replacement systems like the Zimmer Durom cup should contact
a lawyer as quickly as possible to ensure that they stay within the statute of
limitations for their state.



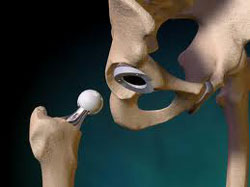











 As a result of the ongoing Zimmer hip lawsuits filed by patients who have experienced side effects as a result of Zimmer hip replacement systems, several settlements have been given out, amounts that could help compensate for costs associated with the hip replacement complications. Medical bills, lost wages, and other expenses will be considered when a settlement is given out.
As a result of the ongoing Zimmer hip lawsuits filed by patients who have experienced side effects as a result of Zimmer hip replacement systems, several settlements have been given out, amounts that could help compensate for costs associated with the hip replacement complications. Medical bills, lost wages, and other expenses will be considered when a settlement is given out. Since August 19, 2008, Zimmer has been offering web-based training to surgeons to highlight important aspects of the Durom Cup design, pre-operative planning that is necessary and detailed instructions on special surgical techniques that are required. They have indicated that they will start making the Zimmer Durom Hip Cup available to doctors after they complete the internet training. Zimmer allowed approximately 12,000 people in the United States to receive their Durom Cup, even though they knew or should have known that doctors were not provided with adequate warnings and instructions about the surgical techniques required to avoid failure of the hip replacement parts. According to Zimmer’s own estimates, some doctors experienced Zimmer hip implant failure rates as high as 5.7% with the Durom Cup.
Since August 19, 2008, Zimmer has been offering web-based training to surgeons to highlight important aspects of the Durom Cup design, pre-operative planning that is necessary and detailed instructions on special surgical techniques that are required. They have indicated that they will start making the Zimmer Durom Hip Cup available to doctors after they complete the internet training. Zimmer allowed approximately 12,000 people in the United States to receive their Durom Cup, even though they knew or should have known that doctors were not provided with adequate warnings and instructions about the surgical techniques required to avoid failure of the hip replacement parts. According to Zimmer’s own estimates, some doctors experienced Zimmer hip implant failure rates as high as 5.7% with the Durom Cup.

