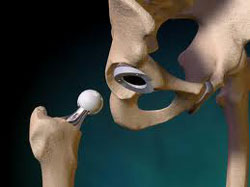
The Zimmer Durom Cup hip implant was first introduced in the United
States in 2006, with a design that was supposed to avoid many problems
associated with traditional hip replacement components, such as instability,
limited range of motion and wear of the bearing. However, shortly after it was
introduced, concerns emerged about a high number of Zimmer hip replacement failures
involving the Durom Cup, where the component loosened and required
revision surgery. A temporary Zimmer hip recall of the Durom Cup implant was issued in July 2008,
The Zimmer Durom Cup hip implant was first introduced in the United
States in 2006, with a design that was supposed to avoid many problems
associated with traditional hip replacement components, such as instability,
limited range of motion and wear of the bearing. However, shortly after it was
introduced, concerns emerged about a high number of Zimmer hip replacement failures
involving the Durom Cup, where the component loosened and required
revision surgery. A temporary Zimmer hip recall of the Durom Cup implant was issued in July 2008, Wednesday, October 26, 2011
Ballooning Fund For Zimmer Hip Lawsuits Prepared to Burst
 The Zimmer Durom Cup hip implant was first introduced in the United
States in 2006, with a design that was supposed to avoid many problems
associated with traditional hip replacement components, such as instability,
limited range of motion and wear of the bearing. However, shortly after it was
introduced, concerns emerged about a high number of Zimmer hip replacement failures
involving the Durom Cup, where the component loosened and required
revision surgery. A temporary Zimmer hip recall of the Durom Cup implant was issued in July 2008,
The Zimmer Durom Cup hip implant was first introduced in the United
States in 2006, with a design that was supposed to avoid many problems
associated with traditional hip replacement components, such as instability,
limited range of motion and wear of the bearing. However, shortly after it was
introduced, concerns emerged about a high number of Zimmer hip replacement failures
involving the Durom Cup, where the component loosened and required
revision surgery. A temporary Zimmer hip recall of the Durom Cup implant was issued in July 2008, Friday, October 21, 2011
Zimmer Hip Lawyers Fight for Different Types of Damages
Papaccio v. Zimmer
Holdings, in the U.S. District Court in the Middle District of Florida on
July 11, 2011 asked for declaratory relief. Declaratory relief is a judge's determination of the parties' rights under a
contract or a statute often requested for information in a lawsuit over a
contract. The theory is that an early resolution of legal rights will resolve
some or all of the other issues in the matter. Over 12,000 people
in the United States have had a Zimmer Durom Cup implanted during their hip
replacement surgery. Hundreds of these people could experience Zimmer hip failure due to loosening
of the component and the need for additional surgeries which was caused by the
negligence of Zimmer Holdings, Inc. They introduced a new product without
providing adequate warnings or instructions about the proper use and surgical
techniques required leading to many hip replacement patients looking to file a Zimmer hip lawsuit.
Wednesday, October 19, 2011
Large Fund Set Up By Zimmer To Settle Lawsuits
 The Zimmer Durom Acetabular Component is
a newer type of artificial hip part which is designed for use in combination
with Zimmer’s Metasul Metal-on-Metal Tribological Solution Large Diameter Heads
(LDH). The Zimmer Durom cup hip replacement is a monoblock of cobal chromium alloy which was
introduced in Europe in 2003 and approved in the United States in 2006. In May
2008, Zimmer sent a letter to healthcare providers indicating that they were
initiating an investigation into the complaints of Zimmer Durom Cup complications. After reviewing data on over 3,100 cases, Zimmer suspended sales
of the Durom artificial hip component in July 2008. All federal lawsuits over problems with Zimmer NexGen knee
replacements have been consolidated for pretrial proceedings as part of an
MDL, or multidistrict litigation, which will be centralized in the U.S.
District Court for the Northern District of Illinois.
The Zimmer Durom Acetabular Component is
a newer type of artificial hip part which is designed for use in combination
with Zimmer’s Metasul Metal-on-Metal Tribological Solution Large Diameter Heads
(LDH). The Zimmer Durom cup hip replacement is a monoblock of cobal chromium alloy which was
introduced in Europe in 2003 and approved in the United States in 2006. In May
2008, Zimmer sent a letter to healthcare providers indicating that they were
initiating an investigation into the complaints of Zimmer Durom Cup complications. After reviewing data on over 3,100 cases, Zimmer suspended sales
of the Durom artificial hip component in July 2008. All federal lawsuits over problems with Zimmer NexGen knee
replacements have been consolidated for pretrial proceedings as part of an
MDL, or multidistrict litigation, which will be centralized in the U.S.
District Court for the Northern District of Illinois. Wednesday, October 5, 2011
Zimmer Hip Replacement Problems: Device or Surgeon?
 Zimmer is making claims that there have been an excessive
number of Zimmer hip failures associated with their Durom Cup knee replacement system not
because the device itself is faulty, but because surgeons who are implanting
the device in patients are doing so incorrectly. They also cite other possible
complications that could have an impact on the viability of the device –
infection, dislocation, trauma, bone fractures, and metal hypersensitivity
could, claims Zimmer, make a medical device such as the Durom Cup more prone to
failure. Despite these claims, a class action lawsuit has been filed in Canada
that involves every one of the almost 5,000 Canadians who were fitted with a
Zimmer Durom Cup hip replacement system.
Zimmer is making claims that there have been an excessive
number of Zimmer hip failures associated with their Durom Cup knee replacement system not
because the device itself is faulty, but because surgeons who are implanting
the device in patients are doing so incorrectly. They also cite other possible
complications that could have an impact on the viability of the device –
infection, dislocation, trauma, bone fractures, and metal hypersensitivity
could, claims Zimmer, make a medical device such as the Durom Cup more prone to
failure. Despite these claims, a class action lawsuit has been filed in Canada
that involves every one of the almost 5,000 Canadians who were fitted with a
Zimmer Durom Cup hip replacement system.
Subscribe to:
Comments (Atom)